Abstract
Background: Dara, a human IgGκ monoclonal antibody that targets CD38, is approved in combination with bortezomib, melphalan, and prednisone (VMP) for the treatment of newly diagnosed (ND) MM. CyBorD is another commonly used immunomodulatory drug-sparing regimen for MM. We evaluated the safety and efficacy of dara-CyBorD and administered the first dara infusion as a split dose over 2 days in pts with NDMM or relapsed MM (RMM) after 1 prior line of therapy.
Methods: This is an ongoing, multicenter, single-arm, open-label, phase 2 study conducted at US community oncology centers in pts aged ≥18 years with documented MM per IMWG criteria; measurable disease; ECOG performance score (PS) of 0-2; and ≤1 prior line of therapy. Pts received 4-8 cycles (C) of dara-CyBorD (oral cyclophosphamide 300 mg/m2 on Days 1, 8, 15, and 22; subcutaneous bortezomib 1.5 mg/m2 on Days 1, 8, and 15; and oral or IV dexamethasone 40 mg weekly) every 28 days. Dara was administered at 8 mg/kg IV in 500 ml on Days 1 and 2 of C1, 16 mg/kg weekly from C1D8 through C2, 16 mg/kg every 2 weeks (q2w) for C3-6, and 16 mg/kg q4w for C7-8. After induction, pts could undergo autologous stem cell transplantation (ASCT). All pts receive 12 cycles of maintenance dara 16 mg/kg IV q4w. The primary endpoint was the proportion of pts achieving very good partial response or better (VGPR+) after 4 induction cycles using a computer algorithm based upon IMWG response criteria.
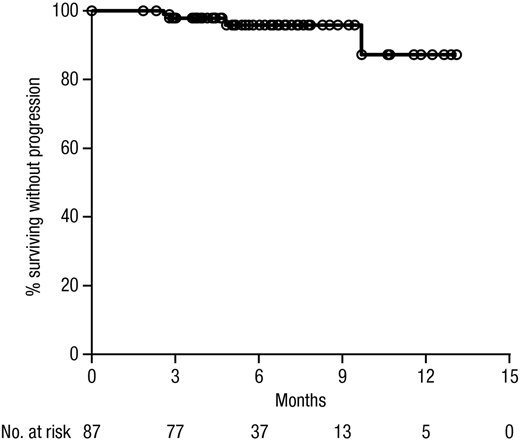
Results: A total of 101 (87 ND, 14 RMM) pts were enrolled; 100 (86 ND, 14 RMM) pts received at least 1 dose of study treatment. Median age was 63 years (63 ND, 68 RMM); most pts were white (81%), male (64%), had ECOG PS 0-1 (94%), and had IgG (57%) or IgA (17%) MM; 35% of pts had high-risk cytogenetics defined as del(17p), t(4:14), or t(14;16). Eighty-two ND pts completed at least 4 induction cycles, 55 at least 6 cycles, and 26 the maximum of 8 cycles; 28 ND pts underwent ASCT by the data cutoff date. After 4 induction cycles, 44% of ND pts achieved VGPR+ (5% CR) with an overall response rate (ORR) of 79%. The VGPR+ rate (57%), CR rate (14%), and ORR (71%) were similar in RMM pts. At the end of induction (median 6 cycles), the VGPR+ rate, CR rate, and ORR in ND pts were 56%, 9%, and 81%, respectively. With a median follow up of 7.9 months, median PFS and OS were not reached; the 12-month PFS and OS rates were 87% and 99%, respectively, in ND pts. All 100 evaluable pts experienced ≥1 treatment-emergent adverse event (AE). AEs with incidence ≥20% included fatigue, nausea, diarrhea, cough, insomnia, vomiting, constipation, upper respiratory tract infection, dyspnea, headache, and back pain. Grade ≥3 AEs were reported for 56% of pts; the most common (≥10%) was neutropenia. Serious AEs (SAEs) occurred in 21% of pts; the most common (≥2%) were atrial fibrillation, bacteremia, pulmonary embolism, and mental status changes. AEs led to permanent treatment discontinuation in 3% of pts. Infusion reactions (IRs) occurred in 54% of pts, including 49% at C1D1 and 4% at C1D2; 2 Grade 3 IRs (hypertension, anaphylactic reaction) occurred at C1D1; no Grade ≥4 IRs occurred. The most common (≥5%) IRs were chills, cough, dyspnea, nausea, pruritus, and flushing. Median infusion time was 4.5 hours for C1D1, 3.8 hours for C1D2, and 3.5 hours for subsequent doses.
Conclusion: Dara-CyBorD was active and well tolerated in pts with ND and RMM, including pts with high-risk cytogenetics. ORR, VGPR+, and CR rates improved with cycles 5-8 of induction, indicating that longer therapy with dara results in deeper response. Preliminary PFS and OS data in ND pts in the first year are comparable to dara-VMP. The safety profile was consistent with that previously reported for dara, with no new safety signals observed. Split first daratumumab dosing was feasible, reduced Day 1 infusion time, and resulted in a similar IR rate as previously described for single-dose administration. These findings indicate that dara-CyBorD, using a split-dose first infusion, can be safely administered in the community setting and may be an effective treatment option for pts with MM.
www.clinicaltrials.gov identifier: NCT02951819
Figure 1. Kaplan-Meier estimate of progression-free survival (PFS) among patients with newly diagnosed multiple myeloma.
Yimer:AstraZeneca: Speakers Bureau; Puma Biotechnology: Equity Ownership; Clovis Oncology: Equity Ownership; Celgene: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Epizyme: Equity Ownership; Janssen: Speakers Bureau. Melear:Janssen: Speakers Bureau. Faber:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cardinal Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bensinger:celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau; amgen: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Burke:Gilead: Consultancy; Genentech: Consultancy; Celgene: Consultancy; Abbvie: Consultancy; Bayer: Consultancy; Seattle Genetics: Consultancy, Speakers Bureau; Tempus Labs: Consultancy. Narang:Janssen: Speakers Bureau; Celgene: Consultancy, Speakers Bureau. Stevens:Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Gunawardena:Janssen/ Johnson & Johnson: Employment, Equity Ownership. Lutska:Janssen/ Johnson & Johnson: Employment, Equity Ownership. Qi:Janssen/ Johnson & Johnson: Employment, Equity Ownership. Ukropec:Janssen Scientific Affairs, LLC: Employment. Qi:Janssen Research & Development, LLC: Employment. Lin:Janssen/ Johnson & Johnson: Employment, Equity Ownership. Rifkin:Amgen: Consultancy; McKesson: Equity Ownership; Boehringer Ingelheim: Consultancy; Celgene: Consultancy; EMD Serono: Consultancy; Takeda: Consultancy; Sandoz: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal